
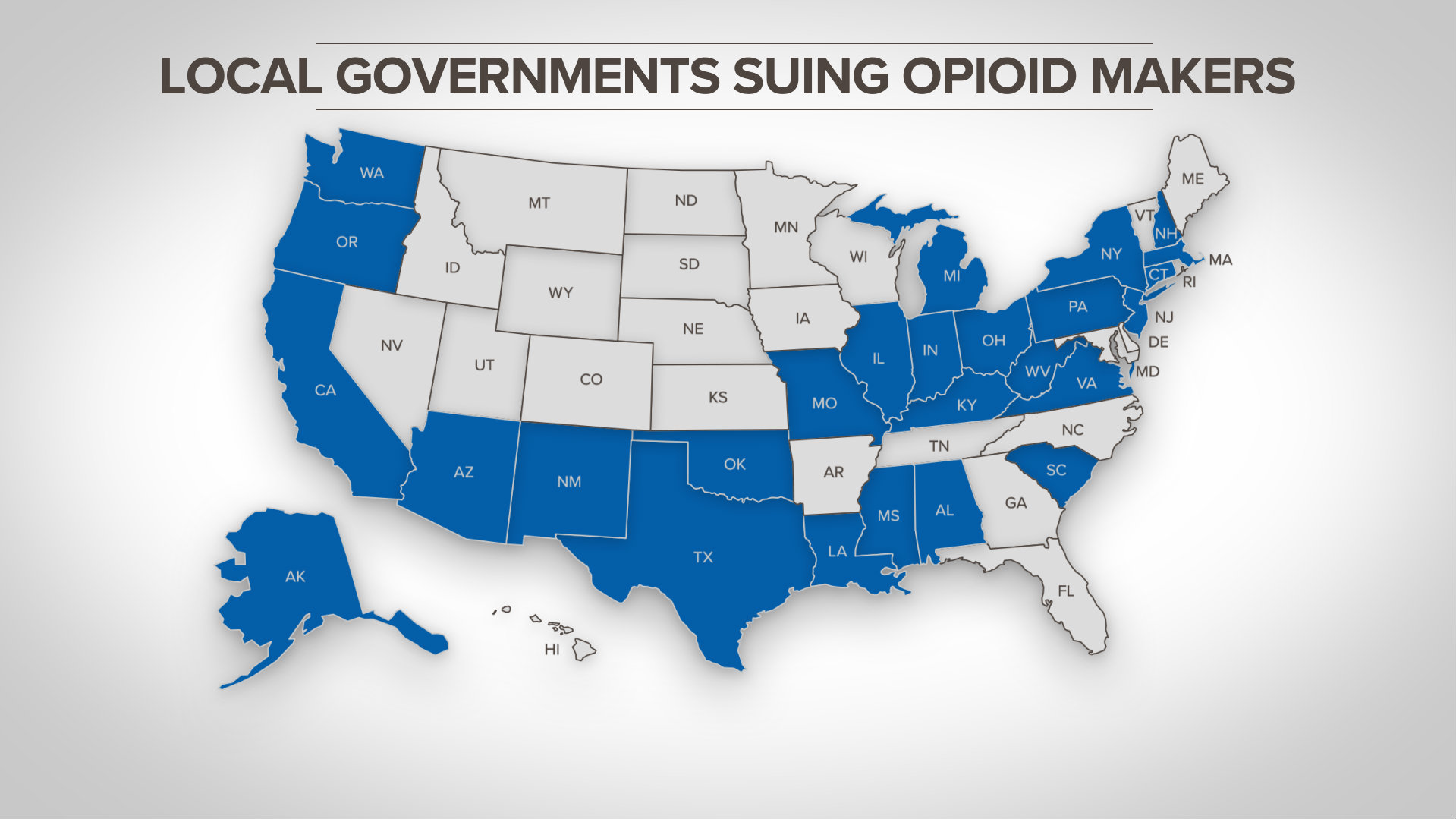
Health Care, Pharmaceuticals and Medical Devices. That law was passed after a fungal meningitis outbreak linked to contaminated steroids manufactured by New England Compounding Center in 2012 that prosecutors say killed 76 people and sickened hundreds more.Ī federal jury in Boston on Wednesday found the former supervisory pharmacist at Massachusetts-based NECC, Glenn Chin, guilty of racketeering and fraud charges, while clearing him of second-degree murder. The Mass Torts, Insurance and Consumer Litigation Group at Skadden has been involved in many of the. Qualitest is a manufacturer of generic pharmaceutical products a wholly owned subsidiary of Endo Pharmaceuticals. Three opioid distributors have settled a lawsuit with New York state to the tune of over 1 billion, according to the office of NY Attorney General Letitia James. The lawsuit comes after FDA Commissioner Scott Gottlieb said in September the agency was working on a new policy that would encourage more compounding pharmacies to register under the Drug Quality and Security Act of 2013. Now, about 50 billion in settlement funds have begun to flow to state governments. In a lawsuit filed in federal court in Washington, the Endo subsidiaries alleged that the FDA had improperly authorized the bulk compounding of hundreds of drugs, including “essentially a copy” of Endo’s blood pressure drug Vasostrict. Aneri Pattani Enlarge this image States filed lawsuits against corporations involved in the opioid crisis.

Food and Drug Administration of ignoring key components of a law passed after a deadly 2012 meningitis outbreak linked to a compounding pharmacy. Endo recently settled a related qui tam whistleblower lawsuit, where the company admitted that the product labeling for the Tablets included dosage information, which stated the supplements contained 1.0 mg, 0.5 mg, or 0.25 mg of fluoride, respectively and that the the multivitamin tablets contained approximately 44 of the fluoride ion rec. Food and Drug Administration of ignoring key components of a law passed after a deadly 2012 meningitis outbreak.
/arc-anglerfish-arc2-prod-pmn.s3.amazonaws.com/public/OVDZMWWEUVEMDOWX6O2KTMS2TQ.jpg)
BOSTON (Reuters) - Endo International Plc said two units filed a lawsuit on Thursday accusing the U.S. Endo International Plc said two units filed a lawsuit on Thursday accusing the U.S.


 0 kommentar(er)
0 kommentar(er)
